Research interests and activity:
The topic of his research work is concentrated on fundamental and applied problems of cellulose chemistry, papermaking chemistry and science, i.e. wet-end chemistry, coating chemistry, white water recirculation, paper converting etc., but recently epimolecular chemistry is centre of his interest. Milichovský has written or contributed to more as 130 scientifically and technical papers (cited more as 160). He has presented a number of papers at numerous conferences, local section meetings, seminars and other industry meetings. A lot of his research works from pulp and paper technology was realized (author or co-author about 100 technical and technological works). He also holds a lot of patents (40), especially for pulp and paper technology. A topic of his research and scientific works was mainly published in these journals:
-
Papír a celulóza (in Czech, English or German)
-
Zellstoff und Papier (in German)
-
Scientific Papers University of Pardubice (in Czech or English)
-
Chimia Drevesiny (in Russian)
-
Kolloidnyj Zhurnal (in Russian)
-
Tappi Journal (in English)
-
Cellulose Chemistry and Technology (in English or German)
-
Papier + Kunststoff Verarbeiter (in German)
-
Przegląd Papierniczy (in Polish or English)
-
Polymer International (in English)
-
Adsorption Science & Technology (in English)
-
Journal of Applied Polymer Science (in English)
-
Journal of Biomaterials and Nanobiotechnology (in English)
-
ISRN Materials Science (in English)
-
Acta Facultatis Xylologiae Zvolen (in Slovak or English)
-
BioResources (in English)
-
etc.
However, his main scientific orientation has been predominantly focused on problems of epimolecular chemistry as evaluation of hypermolecular porosity structures of lignocellulosic materials and their properties (see files 8-12) and water behaviour and interactions with hydrophilic interfaces in macro- and micro-reticular systems as represented by biomaterials predominantly on cellulosic base.
He worked up the SCHL theory and conception of hydration bonds as basic tool of supra- and hyper-molecular chemistry.
Hydration Bonding System – Attractive and Repulsive Hydration Forces
SCHL (Structure Change in Hydration Layers) theory
as most important phenomenon in supra- and hypermolecular chemistry
An increased interest has witnessed in hypermolecular research and application the past 10 years, sparked mostly by technological interests in new and renewable materials on biological basis or similar to biological system and more environmentally friendly and sustainable resources. We are living in era of nanotechnologies, bioengineering, biomaterials, biomanipulation, biopolymers, bioacceptability, biocompatibility etc. The mankind interest has been step by step concentrated into engineering of macro-objects (mechanical engineering) then micro-objects (micro-engineering) and now in nano-objects (nano-engineering) - the world of molecules. Typical nano-world upon highest sophisticated level with endless self-organization and reproducible abilities are living organism – the bio-objects. The bio-world is traditionally environment where we are living. The mankind step by step has researched this bio-world because of the existence reasons. Firstly it is classification of bio-objects, secondly a deeply explanation of their behaviour and thirdly a mechanism of this behaviour on molecular level followed by aim to bioengineered of them.
The bio-objects are composed by two basic biopolymers:
-
cellulose (relative simple polymer, non-moveable bio-objects, e.g. one-year plants, tries etc.)
-
polypeptides (complicated scale of polymers, moveable bio-objects, e.g. animals etc.)
The hydrogen bond is very important in biological systems and for existence of life but presence of water molecules are most pre-requisite for its creation. The creation of hydrogen bond is perhaps the most essential type of bond from the papermaker’s point of view.
The hydrogen bond is very important in biological systems and for existence of life but presence of water molecules are most pre-requisite for its creation. The creation of hydrogen bond is perhaps the most essential type of bond from the papermaker’s point of view.
A lot of unambiguous evidences is known that without water the bio-objects could not be existed and function, however, without the liquid water. The water molecules play a key role in existence of the bio-objects. Without water are no life and no products of life processes. The biopolymers are self-connected with water into hydrogel discontinuities of different consistencies, size, structure and shape forming so the whole body of bio-object. Terms as immobilised water, vicinal water or non-soluble water are connected with this behaviour and the properties of biomaterials. However, the behaviour of mostly bio-objects and other water-based systems we are not be able satisfactory to explain by use of these terms.
For instance, the basic phenomena can be demonstrated as:
-
a connectivity between hydrogen bonding system in paper and paper products and the key role of water during its creation (milled pulp, i.e. refined pulp in dry state has no bonding abilities controversy to beated pulp, i.e. refined pulp in wet state)
-
atypical behaviour of special urea-formaldehyde pre-condensate system during dilution with water (hydrophilic water systems which in concentrated state form optically homogeneous system but getting turbid in diluted state)
-
atypical very interestingly behaviour of high-concentrated homogeneous transparency water system as sodium acetate, honing etc. and called as super molecular liquid – crystallised state transition which by contact with microcrystal of this one only a crystallisation of the whole system is triggered
-
biomimetic systems especially muscles movement although recently electroactive cellulose biomimetic sensor/actuator termed artificial muscles due to their operational similarity to biological muscles was described (Kim J., Yun S., Ounaies Z.: Discovery of Cellulose as a Smart Material, Macromolecules 2006, 39, 4202-4206) but with traditionally irrationally explanation of mechanism of their behaviour based on unrealistic relative slowly migration of sodium ions
-
atypical temperature and composition influence upon rheology of aqueous glues and coating colours distinguishing by existence of peaks upon viscosity – temperature curves
-
etc.
For further development of our knowledge it is crucial to ascertain a mechanism responsible for the performance phenomena mentioned above.
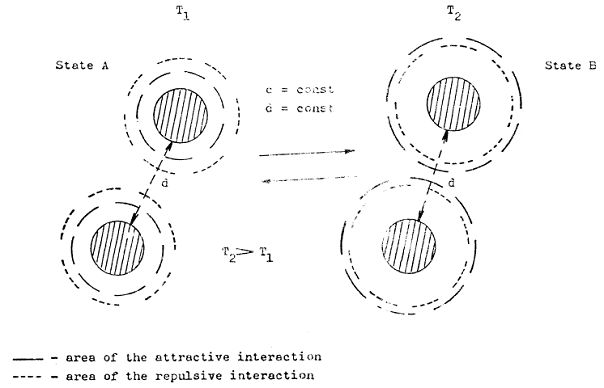
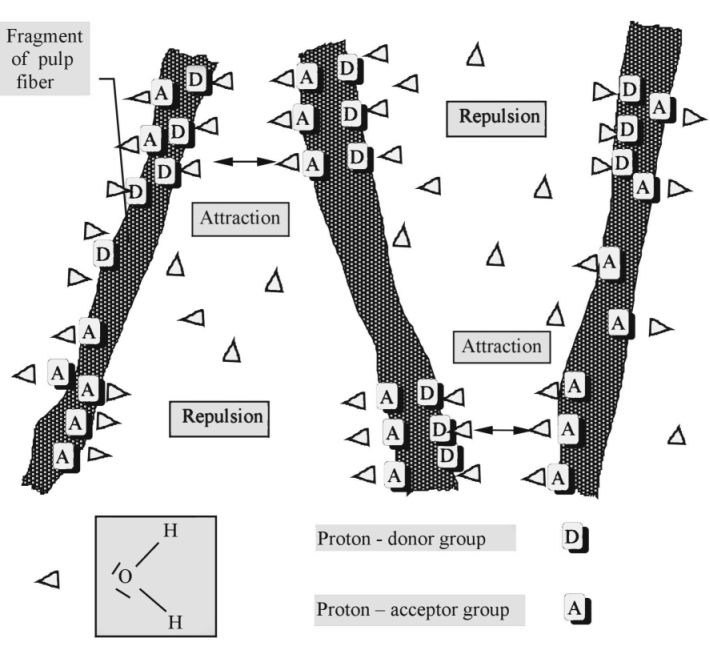
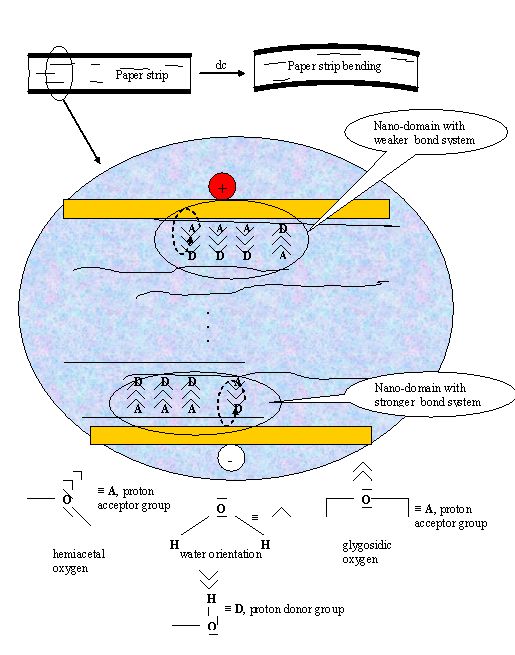
Figure 1 - Schematically representation the concept of immobilised water and hydration bonding system
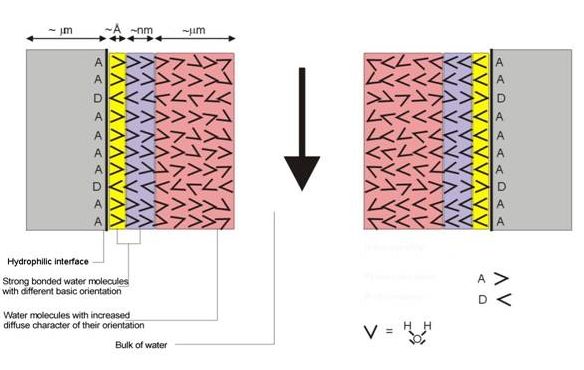
The basic orientation of water molecules in vicinity of nano-locality interfaces with:
- Proton-Acceptor activity A >
- Proton-Donor activity D <
It is no problem to explain satisfactory all of these challenged questions by use of SCHL theory (file 1), the concept of hydration bonding system applied in supramolecular and hypermolecular water systems and practical aspects and behaviour of real highly concentrated water systems (file 2) inclusive. Figure 1 shows schematically the main concept of SCHL theory. Dipole character of water molecule controls its orientation around hydrophilic interface composed with proton-acceptor (A) and proton-donor (D) groups. Due to this fact, different basic orientations of vicinal water molecules around nano-localities of hydrophilic interface are evoked with more and more diffusion character at higher distance. Theoretically, the disappeared distance, i.e. where this oriented structure disappears and is even with bulk of water, is approximately 30 – 80 nm. Logically, mutually interaction of nano-surfaces (depicted in Figure 1) with the same orientation of water molecules are complied with repulsive hydration forces characterized by high tendency to disturb this orientation. By virtually shift of the interfaces depicted at Figure 1, the attractive hydration forces eventually stabile hydration bonding system are evoked, i.e. the attractive hydration forces are prevailed the repulsive ones. The action of hydration forces is strongly influenced by temperature – with increasing temperature the both attractive and repulsive forces decrease but distance of their action increases. Interestingly, at comparable conditions, i.e. predominantly constant interfaces only differentiated with opposite activities of nano-localities and temperature, the distance of repulsive forces action is longer than for the attractive ones.
The study "The Bend+Libration Combination Band Is an Intrinsic, Collective, and Strongly Solute-Dependent Reporter on the Hydrogen Bonding Network of Liquid Water" reports comprehensively how the combination band acts as an intrinsic and collective probe in various chemically and biologically relevant water systems, including salts solutions of varying character, denaturants, osmolytes, crowders, and surfactants that form reverse micelles and micelles.
| go to file list |
Connectivity between hydrogen bonding system in paper and paper products
All aspects of forest and plant based products and the processes by which they are made, are impacted by the relationship between water and the lignocellulosic components of said products. The response of cellulose, hemicelluloses and lignin to moisture (both liquid and vapour) is due almost entirely to the super molecular structure of the biopolymers and the nanoscale structures of the lignocellulosic composites that comprise the wood or plant fibres. Factors such as extractives content and location also play a role. However, most of the response to moisture depends on characteristics of the nanoscale structures in the fibre walls. Primary micro fibrils, which can range in size from about 4 nm – 20nm are composed of cellulose polymer chains arranged in ordered (crystalline) and less ordered (amorphous) regions.
Cellulose is found in plants as micro fibrils (2-20 nm diameter and 100 - 40 000 nm long). These form the structurally strong framework in the cell walls. Cellulose is mostly prepared from wood pulp. Cellulose is also produced in a highly hydrated form by some bacteria (for example, Acetobacter xylinum). Cellulose is a linear polymer of β-1,4-D-glucopyranose units in 4C1 conformation. The fully equatorial conformation of β-linked glucopyranose residues stabilizes the chair structure, minimizing its flexibility (for example, relative to the slightly more flexible α-linked glucopyranose residues in amylase).
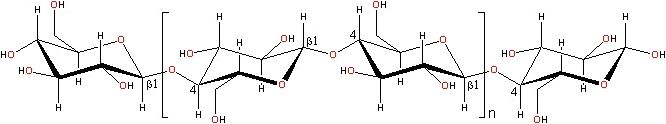
Cellulose preparations may contain trace amounts (approx. 0.3%) of arabinoxylans.
Cellulose is an insoluble molecule consisting of between 2000 - 14000 residues with some preparations being somewhat shorter (see also “practical aspects and behaviour of real highly concentrated water systems” (file 2), or “a new concept of chemistry refining processes” (file 3)). It forms crystals (cellulose Iα) where intra-molecular (O3-HO5' and O6H-O2') and intra-strand (O6-HO3') hydrogen bonds holds the network flat allowing the more hydrophobic ribbon faces to stack. Each residue is oriented 180° to the next with the chain synthesized two residues at a time. Although individual strand of cellulose are intrinsically no less hydrophilic, or no more hydrophobic, than some other soluble polysaccharides (such as amylase) this tendency to form crystals utilizing extensive intra- and intermolecular hydrogen bonding in dry state makes it completely insoluble in normal aqueous solutions (although it is soluble in more exotic solvents such as aqueous N-methylmorpholine-N-oxide (NMNO), approx. 0.8 mol water/mol, then up to 30% by wt cellulose at 100°C ), CdO/ethylenediamine (cadoxen), LiCl/N,N'-dimethylacetamide or near-supercritical water).
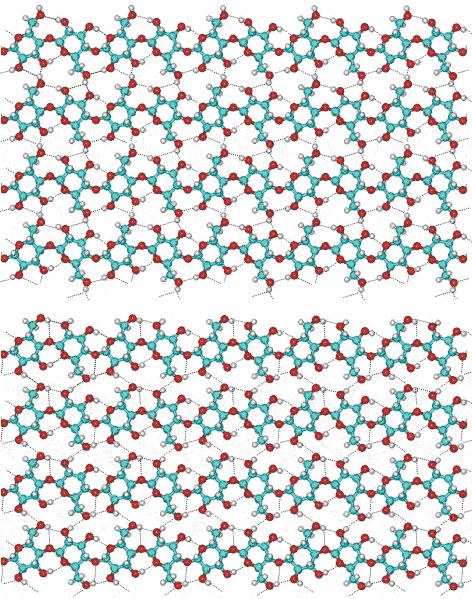
It is thought that water molecules enable the formation of the natural cellulose crystals by helping to align the chains through hydrogen-bonded bridging. This help is connected with action of attractive hydration forces in wet state. Part of a cellulose preparation is amorphous between these crystalline sections. The overall structure is of aggregated particles with extensive pores capable of holding relatively large amounts of water by micro- and nano-capillarity. The natural crystal is made up from metastable Cellulose I with all the cellulose strands parallel and no inter-sheet hydrogen bonding. This cellulose I (that is, natural cellulose) contains two coexisting phases cellulose Iα (triclinic) and cellulose Iβ (monoclinic) in varying proportions dependent on its origin; Iα being found more in algae and bacteria whilst Iβ is the major form in higher plants. Cellulose Iα and cellulose Iβ are interconverted by bending during micro fibril formation and metastable cellulose Iα converts to cellulose Iβ on annealing. If it can be recrystallized (for example, from base or CS2) Cellulose I gives the thermodynamically more stable Cellulose II structure with an antiparallel arrangement of the strands and some inter-sheet hydrogen-bonding.
Swelled bacterial cellulose (ex. Acetobacter xylinum), in its never-dried state with much smaller fibrils (approx. 1%) than from plants, exhibits pseudoplastic viscosity like xanthan gels but this viscosity is not lost at higher temperatures and low shear rates as the cellulose can retain its structure because influence of weak hydration attractive forces.
Schematic representation of origin and action character of repulsive and attractive hydration forces – formation of hydration bond system – among cellulosic fibre materials in water.
Rheosedimentation - principle
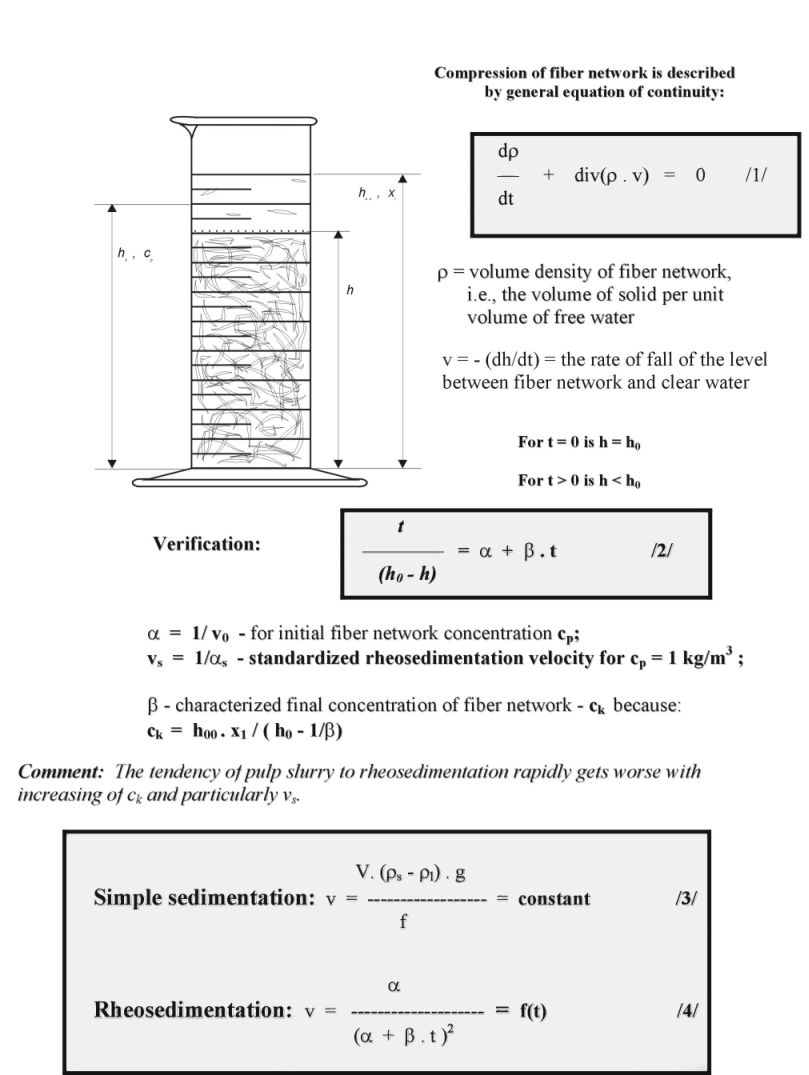
Cellulose has many uses as an anticake agent, emulsifier, stabilizer, dispersing agent, thickener, and gelling agent but these are generally subsidiary to its most important use of holding on to water. Water in low extent penetrates crystalline cellulose but dry amorphous cellulose absorbs water becoming soft and flexible. Some of this water is non-freezing but most is simply trapped. Less water is bound by direct hydrogen bonding if the cellulose has high crystallinity but some fibrous cellulose products can hold on to considerable water in pores and its typically straw-like cavities; water holding ability correlating well with the amorphous (surface area effect) and void fraction (that is, the porosity). As such water is supercoolable, this effect may protect against ice damage. But most important, the cellulose in form of macromolecules, their fragments, elementary strands, microfibrils, fibril, fibrilar strands or at least pulp fibres has hydro-cohesive ability to form structures as paper and paper products but only in water environment. This specifically cellulose behaviour and its insolubility are due to exceptionally ability of cellulose to form hydration bonds followed by creation of hydrogen bonds in dry state. Typically, cellulose in form of pulp fibres creates only in water macro-reticular system characterizing by specifically behaviour denominated as rheosedimentation (file 4). The self-compressing fibre space net formation of low consistency pulp slurry (0,1 w/w %) is a typical for fibres with papermaking abilities because this one is created by action of attractive and repulsive hydration forces finalized with hydrogen bonding system in paper dry form. The connection between formation of hydrogen bond system and hydration bond system among cellulose chains is partly reversible (in amorphous part) and irreversible (in crystalline part of super molecular structure of cellulose) and enable us to change the bonding pulp abilities by refining and beating of ligno-cellulosic materials and their recycling (see also “practical aspects and behaviour of real highly concentrated water systems” (file 2), or “a new concept of chemistry refining processes” (file 3)).
| go to file list |
Atypical behaviour of special urea-formaldehyde pre-condensate
A phenomenon where hydration forces play the main role is the so-called critical degree of dilution (CDD), an untypical case of a limited dilution ability characteristic of some concentrated hydrophilic water systems, e.g. special prepared urea-formaldehyde pre-condensate. The volume of the original pre-condensate (approximately 50% w/w concentration), Vo, being gradually diluted with water or water solutions does not undergo any changes until a moment when the volume of added water or its solution exceeds a critical value, Vk. Now, the originally transparent water system becomes turbid due to precipitated microgel particles.
The value CDD is (Vo + Vk)/Vo. The turbidity is cause by a coacervation of the originally homogeneous system by addition of water or water solutions.
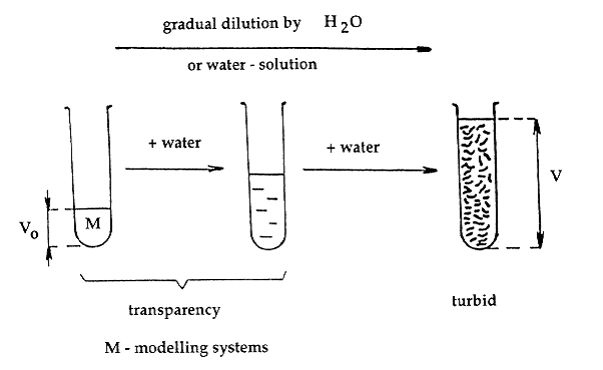
Experimental modelling system of hydration forces (file 5). Evaluation of the effect of hydration forces by means of modelling water system, e.g. urea-formaldehyde pre-condensate.
The super-molecular phenomenon of this behaviour can be simply explained by concept of hydration forces functioning among interacting hydrophilic molecules or their fragments in a water environment. The condition of such behaviour is a presence of hydrated hydrophilic anisometric oligomer molecules. The parts or ends of these molecules influence each other by the attractive hydration forces, while other parts of molecules by the repulsive hydration forces. However, the effect of attractive forces must prevail to that repulsive hydration forces. The uniform distribution of oligomer molecules is prerequisite of following behaviour. When this system is diluted, molecules, their parts and ends are drawn away from each to other and their mutual force action is thus weakened. After exceeding the critical concentration or critical dilution state characterized by critical volume of added water, Vk , the coacervation takes place, owing to fluctuation of the affecting mutual forces and the kinetic energy of interacting oligomer molecules. In this case, the composition and properties of the separated coacervates correspond to the original concentrated system.
The influence rate of the added substances on hydration forces can be evaluated by means of so called hydration factor
g = CDD/CDDo, where CDDo means the CDD value in distilled water. The value g > 1 means that the added substances in form of water solution decrease the attractive forces influence or increase action the repulsive hydration forces and vice versa.
By use of this modelling system it is possible to make a sophisticated characterization the role of hydration in papermaking suspension (file 6), predominantly a new concept of chemistry refining processes (file 3).
| go to file list |
Biomimetic systems especially muscles movement
Recently J. Kim and S. Yun described (see Kim J., Yun S., Ounaies Z.: Discovery of Cellulose as a Smart Material, Macromolecules 2006, 39, 4202-4206) very interesting discovery of cellulose as a smart material that can be used for biomimetic sensor/actuator devices and micro-electromechanical systems. This smart cellulose is termed electroactive paper (EAPap) because it can produce a large bending displacement with low actuation voltage and low power consumption. The above mentioned authors are proposed that electroactive paper is advantageous for many applications such as micro-insect robots, micro-flying objects, micro-electromechanical systems, biosensors, and flexible electrical displays. By use of this phenomenon it is possible also to explain and simulate muscles movement.
The actuation phenomenon of EAPap and its characteristics are illustrated in Figure below. EAPap is made with a cellulose film (cellophane) on which gold electrodes are deposited on both sides. An EAPap actuator was supported vertically in environment chamber
Concept of electroactive paper actuator (EAPap)
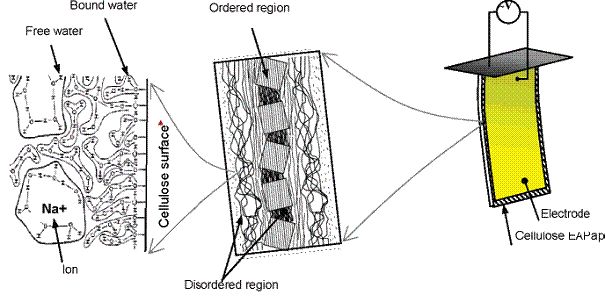
EAPap can be controlled the humidity and temperature. By excitation of voltage application to the actuator a bending deformation is evoked. The tip displacement of the EAPap actuator is dependent on applied electric field, its frequency, EAPap sample thickness and temperature but predominantly on humidity. The humidity affects the displacement, where a high relative humidity leads to a large displacement.
The authors believed that the actuation is due to a combination of two mechanisms: ion migration and dipolar orientation. Cited: “The EAPap material has large regions of disordered cellulose chains, where water molecules can be found attached to hydroxyl groups (see above figure). During the paper making process, sodium ions were injected in the paper fiber. When an external electric field is applied, these ions can be mobile and migrate to the anode. In addition, the molecular motion of free water in disordered region cannot be restricted by the cellulose molecules, and the water molecules can be interacted with ions in the cellulose. In the presence of electric field, the sodium ions surrounded with free water molecules will move to the anode. Selective ionic and water transport across the polymer under electric field results in volumetric changes, which in turn lead to bending. When a dc electric field was applied, the cellulose EAPap actuator was bent to the positive electrode, which confirmed the above explanation. The ambient humidity effect on the EAPap actuator performance is a further evidence of this, where ion transport is facilitated when humidity intake is higher.”
Obviously, at first look, their explanation is wrong and irrational. At least a diffusion of sodium ions to cathode (not anode) is very slowly in comparison with practical observation of actuation.
Explanation according to SCHL theory
An orientation of water molecules in immobilised layers around cellulose macromolecules in stratified structure of EAPap actuator is determined by presence of proton donor groups or proton acceptor groups at their surfaces. The overall film structure and its shape are formed among structural cellulosic units due to both the hydrogen-bonding bridging in dry state and the hydration-bonding bridging in wet state. Extent and intensity of this bonding system is determined by size, concentration and distribution of nano-domains either with the attractive or the repulsive force action, i.e. among interacting opposite nano-surfaces with reversal or identical basic orientation of water molecules, respectively. The basic orientation of water molecule is given by presence of surface proton donor groups or proton acceptor groups of cellulose. Whilst hemiacetal and glycosidic oxygen in cellulose is typical proton-acceptor groups the hydroxyl groups can behave as proton-donor and proton-acceptor groups. Nevertheless, one is supposed that mostly behaviour of hydroxyl groups in cellulosic materials has more a proton-donor character.
In consequence of this preposition, the domains of prevailing hydration-bonding bridging are regularly distributed within cellulosic material with flat formation. In any case of disturbing this distribution, the paper strip curling is evoked because the inner tension equilibrium is broken. As schematically presented in Figure, by application of oriented electric field on cellulosic material in wet state the water molecules in bonding nano-domains contained nearest the electrodes are reoriented. However, reorientation at cathode is different of the reorientation at anode – at anode are reoriented only all the water molecules having been oriented to this pole with hydrogen atoms and at cathode only these ones having been oriented to this pole with oxygen atoms at basic origin state. Moreover, the distribution of attractive forces formed around both the A and D and the D and A nano-centres is not the same – it is supposed a prevailing A - D structure orientation in bonding domains. At this situation, an application of dc electric field is evoked a weaker bond system in layers laying near anode and vice-versa a stronger bond system in layers near cathode. Due to this effect the paper strip gets to bend to anode. Logically, the effect is strongly dependent upon relative humidity, the reorientation of water molecules is independent on diffusion process and it is relatively quickly.
Obviously, by similar effect, but in microscale, a muscles movement is possible to explain. The main preposition – the non-symmetrical distribution of attractive forces formed around both the A and D and the D and A nano-centres.
Schematically representation of water molecules reorientation in nano-localities around electrodes of electric input field.
| go to file list |
Super molecular liquid – crystallised state transition
Principle of phenomenon
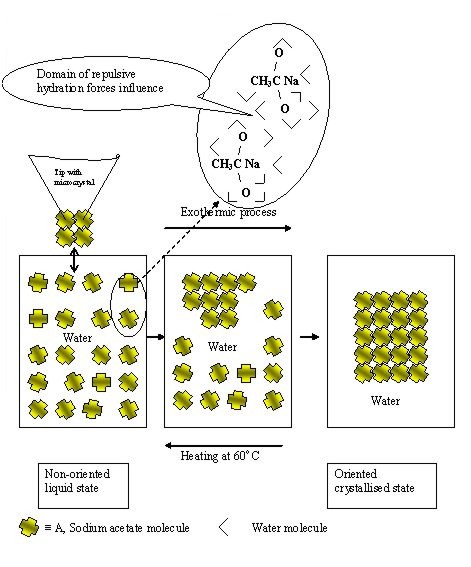
Highly concentrated water liquid system of sodium acetate (approximately 10 w/w % water) with relatively low viscosity (10, mPa.s) is transited at room temperature to crystallised state by contact with microcrystal of this one. The process is full reversible accompanied by release of heat. Heating of whole system at 60oC and careful cooling the liquid state is obtained again.
Explanation
This phenomenon demonstrates a typical behaviour of low molecular systems consisting of molecules substance A, e.g. sodium acetate, homogeneously distributed only in immobilised water. No bulky water is presented, i.e. the individual molecules of A are mutually separated by water molecules penetrating homogeneously the whole system and preventing them to collide finished by their crystallisation. According to SCHL theory (file 1) (see also “Experimental modelling system of hydration forces” (file 5)), this behaviour is possible due to prevailing repulsive hydration forces because equal orientation of water molecules at interfaces around molecules of substance A. Due to weak prevailing hydration repulsive forces, contacting this liquid system with microcrystal of substance A the process of reorientation of molecules A into more stabile more oriented crystalline state is triggered accompanied by heat releasing because the entropy of system decreases. The process takes place step by step by “domino” mechanism through the whole system. With increasing of water concentration the system is also more stable, with lower viscosity and fast expatiation of crystallization.
Schematically representation of molecular heat accumulator.
| go to file list |
Atypical temperature and composition influence upon rheology of aqueous glues and coating colours
Formerly we observed a complicated shape of viscosity – temperature curves of some aqueous glues and coating colours having one or more peaks, i.e. with one or more maximums and minimums. The viscosity - temperature behaviour of these mixtures changes significantly with any change in their composition and depends on shear rate as well. It was observed temperature and composition influence upon rheology of aqueous glues and coating colours (file 7). This behaviour is observable not only in mixtures containing starch or starch derivates.
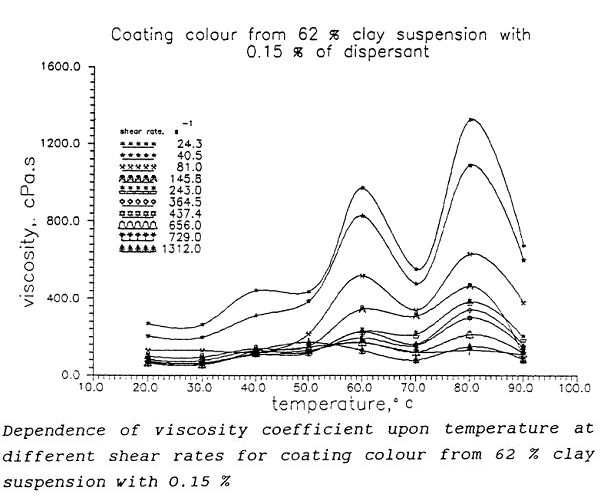
This anomalous behaviour is possible explained only by means of concept the hydration bonding system among interacting particles of dispersion. It seems that both the concentration and the composition play important role in temperature – flow behaviour of these concentrated hydrated hydrophilic systems but the complicated temperature dependences are determined by amount, character and strength of bonds in three-dimensional structure among sites at interacting hydrated hydrophilic phase interfaces of components thus forming coating colours and glues. The explanation of this phenomenon is based on relative simple idea of mosaic type of nano-sites distribution on interacting planes consisting of two types of qualitatively different sites. Both sorts of nano-sites have a common property – their behaviour strongly depends upon temperature. The system achieves a minimum potential energy if attractive forces prevail between interacting planes and a maximum if only repulsive forces exist. At dynamic conditions, when the mutual movement of interacting planes is controlled, the resulting potential energy of steady state is defined by the prevailing force action. At a constant composition of surfaces, they are given by temperature and distance between interacting planes, i.e. by the concentration of interacting nano-sites. The effect of hydration forces belongs among the physical or colloidal intermolecular forces and depends strongly upon temperature. They disappear with rising temperature, but at equal conditions, the repulsive forces are effective over a greater distance. These differences appear important during interactions of surfaces in which repulsive and attractive hydration forces are affected simultaneously. As model predicts, at this situation may exist a temperature at which prevailing attractive forces are functioning whilst at another temperature prevail repulsive hydration forces.
Schematic representation of isopotentials around interacting particles of mosaic type distribution of hydration forces activity – an influence of temperature.